2023 Volume 48 Issue 3 Pages 149-159
2023 Volume 48 Issue 3 Pages 149-159
Reportedly, antibiotics, which are frequently prescribed in children, have long-term effects owing to gut microbiota dysregulation. Tosufloxacin tosilate hydrate (TFLX) is the first orally administered new quinolone with high efficacy and broad-spectrum action approved as an antibacterial agent for pediatric use in Japan. However, studies on the effects of its early-stage administration are limited. Therefore, we aimed to analyze the later effects of its developmental administration by monitoring growth rate, neurobehavior, and gut microbiota in mice. The TFLX was administered via drinking water at a dose of up to 300 mg/kg for two consecutive weeks during the developmental period (4–6 weeks of age) or adulthood (8–10 weeks of age). Thereafter, the body weights of the mice were measured weekly to monitor growth rate. Behavioral tests were also conducted on 11–12-week-old mice to examine the neurobehavioral effects of the treatment. Further, to examine the effects of the treatment on microbiota, fecal samples were collected from the rectum of mice dissected at 12 weeks of age, and 16s rRNA analysis was conducted. Our results showed increased body weights after TFLX administration, without any long-term effects. Behavioral analysis suggested alterations in anxiety-like behaviors and memory recall dysregulation, and gut microbiota analysis revealed significant differences in bacterial composition. These findings indicated that TFLX administration during the developmental period affects mice growth rate, neurobehavior, and gut microbiota structure. This is the first study to report that TFLX is potentially associated with the risk of long effects.
Worldwide, antibiotics are often prescribed to children and adults for infectious diseases, such as otitis media, pneumonia, and influenza. Notably, they are frequently prescribed to children aged 1–5 years (Kinoshita et al., 2019). However, it has been observed that early-life antibiotic administration causes obesity (Cho et al., 2012; Cox et al., 2014) and allergic diseases (Farooqi and Hopkin, 1998; McKeever et al., 2002) with long-term effects. Antibiotics also disrupt the gut microbiome structure, and many of their long-term effects are thought to be caused by these changes or disturbances in the gut microbiota.
The interaction between the brain and the gut (the “brain-gut interaction”) has attracted widespread attention. Specially, the central nervous system communicates with the gastrointestinal tract via the brain-gut axis, and this bilateral communication involves neural-, endocrine-, and immune homeostatic mechanisms (Skonieczna-Żydecka et al., 2018). Further, gut bacteria are increasingly receiving attention as carriers of such communication, and the concept of the “brain-gut-microbiome axis” has been proposed (Cryan and Dinan, 2012). Reportedly, gut bacteria regulate the hypothalamic-pituitary-adrenal (HPA) axis in response to neurotrophic factors in the cortex and hippocampus (Sudo et al., 2004). There are several reports of brain diseases caused by gut bacteria. For example, 4-ethylphenylsulfate (a uremic substance produced by gut bacteria) is responsible for autism-like symptoms in mouse models (Hisao et al., 2013). Additionally, using a mouse model, it was observed that short-chain fatty acids (SCFAs) produced by gut bacteria aggravate movement disorders in Parkinson’s disease (Sampson et al., 2016). Gut bacteria are involved in homeostatic systems via various related substances, nervous system pathways, and their metabolites.
Tosufloxacin tosilate hydrate (TFLX) is a new quinolone antibacterial agent with a broad antibacterial spectrum against gram-positive, gram-negative, and anaerobic bacteria (Sato et al., 2005). It was the first new quinolone approved for the treatment of bacterial pneumonia and acute otitis media in pediatric patients in Japan (Matsumura et al., 2013). Its efficacy with reported to pneumonia and otitis media is 96.1% (224/254 cases) and 96.0% (430/448 cases), respectively (Kamiyama et al., 2017), and its side effects, which may include gastrointestinal problems, such as diarrhea, are not severe and recovery is quick, indicating that the product is safe.
To the best of our knowledge, studies on the effects of TFLX prescriptions at an early age are limited. To enhance understanding in this regard, in this study, we analyzed the effects of TFLX administered during the developmental period on growth rate, neurobehavior, and gut microbiota in later life in mice. Specially, the differences in the effects of TFLX administration at different time on body weight, behavior, and gut bacterial composition were compared. Our results may be useful for assessing the risk of administering TFLX at an early age and in clarifying the mechanism of its toxicity.
Three-week-old male C57BL/6N mice were purchased from Japan SLC (Shizuoka, Japan) and maintained in a room at 24 ± 1°C and 60 ± 10% humidity and a 12-hr/12-hr light/dark cycle for one week prior to treatment. During this period, the mice were provided free access to food (MF diet; Oriental Yeast Co. Ltd., Tokyo, Japan) and water. Thereafter, the mice were divided into three groups (n = 10 per group), namely the control, early TFLX administration (TFLX- I), and late TFLX administration (TFLX-II) groups. Tosufloxacin tosilate hydrate purchased from Wako (Osaka, Japan), was dissolved in 624 ppm N, N-dimethylformamide (DMF) (Nacalai Tesque, Kyoto, Japan). The DMF concentration (4.72 mg/day) was lower than the permitted daily exposure (PDE) dose (8.8 mg/kg). The concentration of tosufloxacin tosilate hydrate was based on the Non Observed Adverse Effect Level (NOAEL) in repeated oral administration studies in juvenile rats (Furubo et al., 2010). Thus, the maximum daily intake was set at 300 mg/kg, assuming that the drinking water volume of mice was 8 mL/day. To match the developmental period in which antibiotics are frequently prescribed in humans, the TFLX-I group received TFLX in DMF solution at 4–6 weeks of age (developmental period), and only the DMF solution via their drinking water at 8–10 weeks of age. For the TFLX-II group, the mice received only the DMF solution at 4–6 weeks of age, and at 8–10 weeks of age (adulthood), they received TFLX in the DMF solution. The mice in the control group were administered only DMF solution at 4–6 and 8–10 weeks of age via their drinking water. After these treatments, a mouse behavioral test battery was performed when the mice were 11–12 weeks of age. All the animals were handled daily for 1 min for a period of week before this test. Fig. 1 depicts the schematic workflow of the experiment. All the animal care and experimental procedures were conducted in accordance with the protocols approved by the Tohoku University Institutional Animal Care and Use Committee.

Study design. White, administration of drinking water only; gray: administration of N, N-dimethylformamide (DMF) in drinking water; black, administration of TFLX in DMF in drinking water.
The behavioral test battery, consisting of an open field test (OF), light/dark transition test (LD), and contextual/cued fear conditioning test (FZ), was performed as previously described (Saito et al., 2019). Image analysis (Image OF2, Image LD2, and Image FZ2; O’Hara and Co., Ltd., Tokyo, Japan) was performed using the public domain ImageJ program. Further, all the experiments were performed between 10:00 and 14:30 with 10 mice per group. The background noise level during the trials was approximately 50 dB. The apparatus was cleaned with water and wiped off after each trial.
Open field testGeneral locomotor activity was measured for 10 min using an open-field apparatus consisting of a white plastic material with dimension 50 cm × 50 cm × 30 [H] cm. The LED light system used was placed 50 cm above the center of the field (50 lx at the center of the field). Thereafter, the behavior of the mice was recorded using a charge-coupled device (CCD) camera positioned above the center of the OF apparatus. Image OF2 was obtained to measure the total distance traveled (cm), total number of movements, average moving speed (cm/sec), time spent in the central area (30% of the field) (sec), total duration of movement (sec), average speed of total test time (cm/sec), distance per movement (cm), and duration covered per movement (sec).
Light/dark transition testThe light/dark transition test apparatus comprised the use of a cage (21 cm × 42 cm × 25 [H] cm) divided into two chambers by a partition with an opening. One chamber consisted of white plastic material that was brightly lit (250 lx, light area), while the other chamber was made up of a black plastic material that was dark (5 lx, dark area). A mouse was placed in the dark chamber and allowed to move freely between the two chambers through the opening for 5 min. Behavior was recorded using a CCD camera positioned above each chamber. Image LD2 allowed the measurement of the total distance in covered the light chamber (cm), total distance covered in the dark chamber (cm), time spent in the light chamber (sec), time spent in the dark chamber (sec), the total number of transitions, and latency to initially move to the light area (sec).
Contextual/cued fear conditioning testThe apparatus consisted of a conditioning chamber (or test chamber) (17 cm × 10 cm × 10 [H] cm) made from a clear plastic material with a ceiling. The chamber floor was made from stainless steel rods (2 nm diameter) spaced 5 mm apart to provide an electrical foot shock to the mouse. The inner wall of the chamber was covered with black and white plastic stripes, and the LED light system was placed approximately 50 cm above the chamber (50 lx at the center of the floor). Thereafter, the mice were individually placed in the conditioning chamber during the conditioning trial for 90 sec. This was followed by the administration of three tone-shock pairings (30 sec of tone at 65 dB, directly followed by 2 sec of 0.08 mA electric shock), each separated by a time interval of 120 sec. In the next step, the mice were returned to their home cages, and 2 days later, they were returned to the conditioning chamber for 6 min without a tone or shock as a contextual fear test. Two days later, they were placed in a novel chamber with a different design without plastic, black and white stripes, or stainless-steel rods for the cued fear test. After 3 min, a conditioning tone was presented for 3 min (without shock). Behavior was then recorded using a CCD camera positioned above the center of the chamber. Further, the freezing response of the mice (a consecutive 2-sec period of immobility) was measured using Image FZ2, and the freezing rate (%) was calculated as follows: [freezing/session time] × 100.
Sample collectionBody weight was measured weekly at 3–12 weeks of age, and at 12 weeks of age, the mice were sacrificed after the behavioral test battery. Fecal samples were collected from the rectum of the mice and stored in sterile tubes at −80°C until analysis. Further, the brains were surgically removed, fixed in methacarn solution (methanol/chloroform/acetic acid at 6:3:1) or 10% formaldehyde neural buffer solution (Nacalai Tesque, Kyoto, Japan), treated with ethanol and xylene, embedded in paraffin, and sectioned into 8-μm sagittal sections. Thereafter, they were mounted on glass slides and examined.
Histological analysisSections were deparaffinized with xylene, rehydrated with ethanol (100%, 95%, 90%, 80%, and 70% gradually), and rinsed with distilled water. This was followed by staining their nuclei with hematoxylin and the staining of the cytoplasm with eosin. The sections were then dehydrated with ethanol, clarified with xylene, and mounted for examination. Images were acquired using a BX63 optical microscope and analyzed using cellSens software (Olympus, Tokyo, Japan).
Immunohistochemical analysisSections were deparaffinized with xylene, rehydrated with ethanol, rinsed with distilled water, and incubated with HistoVT One (Nacalai Tesque, Kyoto, Japan) at 90°C for 30 min. Thereafter. They were kept for 1 hr at 4°C in Blocking One (Nacalai Teque, Kyoto) followed by incubation overnight at 4°C with primary antibodies. The following primary antibodies were used: mouse monoclonal anti neurofilament medium (NF; Abcam, Cambridge, UK; ab7794; diluted 1:400), rabbit polyclonal anti microtubule-associated protein 2 (MAP2; Proteintech, Rosemont, IL, USA; 17490-1-AP; diluted 1:250), mouse monoclonal anti alpha tubulin (α-tubulin; Santa Cruz Biotechnology, Dallas, TX, USA; diluted 1:100), rabbit polyclonal anti doublecortin (DCX; Abcam, Cambridge, UK; ab18723; diluted 1:400), goat polyclonal anti SRY-related HMG-box 2 (SOX2; Novus Biologicals, Littleton, CO, USA; diluted 1:400). After washing with phosphate-buffered saline, immunoreactive elements were visualized following treatment with Alexa Flour 488-labeled anti-mouse, anti-rabbit or anti-goat and Alexa Fluor 555-labeled anti-mouse or anti-rabbit secondary antibodies (Invitrogen, Walthan, MA, USA; diluted 1:1000) for 2 hr at 4°C. Nuclei were stained with Hoechst 33342 (Nacalai Tesque, Kyoto, Japan; diluted 1:5000). At all dilutions, antibodies or Hoechst 33342 were added to a mixture of Blocking One and phosphate buffered saline. Images were then obtained using a FV3000 confocal laser scanning microscope (Olympus, Tokyo, Japan).
Microbiota analysisThe fecal DNA extraction procedure and 16s rRNA gene amplicon (V3-V4 regions) sequencing were performed according to a previously published protocol (Islam et al., 2022). Additionally, demultiplexed raw sequences were obtained from the BaseSpace Sequence Hub (Illumina, San Diego, CA, USA), and the sequences were further analyzed using the QIIME 2 bioinformatics pipeline (version 2020.2) (Bolyen et al., 2019; Islam et al., 2022).
Statistical analysesStatistical analyses were realized by performing Dunnett’s or Tukey–Kramer’s multiple comparison test for parametric data and the Kruskal–Wallis test for nonparametric data according to the protocol. Data were expressed as the mean ± standard error (S.E), statistical significance was set at P < 0.05. In addition, a P-value < 0.1 was considered a statistically significant trend (†P < 0.1). All the statistical analyses were performed using KyPlot 6.0 version 6.0.2 (KyensLab Inc., Tokyo, Japan).
Compared with mice in the control group, those in the TFLX-I group exhibited significant weight gain at 6 weeks of age (control, 20.01 ± 0.98 g; TFLX-I, 21.96 ± 0.80 g; TFLX-II, 19.95 ± 1.25 g), 7 weeks of age (control, 18.93 ± 1.12 g; TFLX-I, 20.67 ± 1.57 g; TFLX-II, 18.39 ± 1.74 g), and 9 weeks of age (control, 19.98 ± 1.33 g; TFLX-I, 21.92 ± 2.04 g; TFLX-II, 19.28 ± 1.72 g). However, no significant differences were observed in this regard for mice in the TFLX-II group at any age. Further, there were no significant differences in weight between both TFLX administration groups before (control, 20.74 ± 1.64 g; TFLX- I, 22.04 ± 2.38 g; TFLX- II, 19.76 ± 1.65 g) and after the behavioral test battery compared with the control group (control, 24.56 ± 1.12 g; TFLX-I, 26.21 ± 1.14 g; TFLX-II: 24.61 ± 1.43 g) (Fig. 2, Table 1).

Growth rate. Weekly weight gain from 3–12 weeks of age (after the behavioral test battery). Data were analyzed using Dunnett’s multiple comparisons test and are expressed as the mean ± S.E. *P < 0.05, ***P < 0.001 compared with the control group (n = 10 per group).
| Body weight (g) | ||||||
|---|---|---|---|---|---|---|
| Treatment groups | Before behavioral test battery (11 weeks) | After behavioral test battery (12 weeks) | ||||
| Control | 20.74 | ± | 0.52 | 24.56 | ± | 0.35 |
| TFLX-I | 22.04 | ± | 0.75 | 26.21 | ± | 0.36 |
| TFLX-II | 19.76 | ± | 0.52 | 24.61 | ± | 0.45 |
Data were analyzed using Dunnett’s multiple comparison test and are expressed as the mean ± S.E.
The OF test revealed that there were no significant difference between the TFLX administration groups and the control group with respect to the total distance, average moving speed, and total center region time (total distance: control, 3707.6 ± 179.4 cm; TFLX-I, 3217.3 ± 185.3 cm; TFLX-II, 3395.5 ± 269.8 cm; average moving speed: control, 11.2 ± 0.3 cm/sec; TFLX-I: 10.8 ± 0.2 cm/sec; TFLX-II: 10.7 ± 0.4 cm/sec; total center region time: control, 77.6 ± 10.7 sec; TFLX-I, 60.8 ± 7.3 sec; TFLX-II, 76.0 ± 12.8 sec) (Fig. 3A, C, and D, respectively). The number of movement episodes was significantly lower for mice in the TFLX-I and TFLX-II groups compared with that observed for the control (control, 196.7 ± 4.6; TFLX-I, 172.7 ± 4.8; TFLX-II, 175.3 ± 5.8) (Fig. 3B). Furthermore, mice in the TFLX-I group showed a significantly smaller moving distance than those in the control group within the initial period (30–60 sec) when considering the moving distance with time (control, 253.3 ± 12.7 cm; TFLX-I, 141.7 ± 24.2 cm; TFLX-II, 244.6 ± 34.2 cm) (Fig. 3E, 3E′).
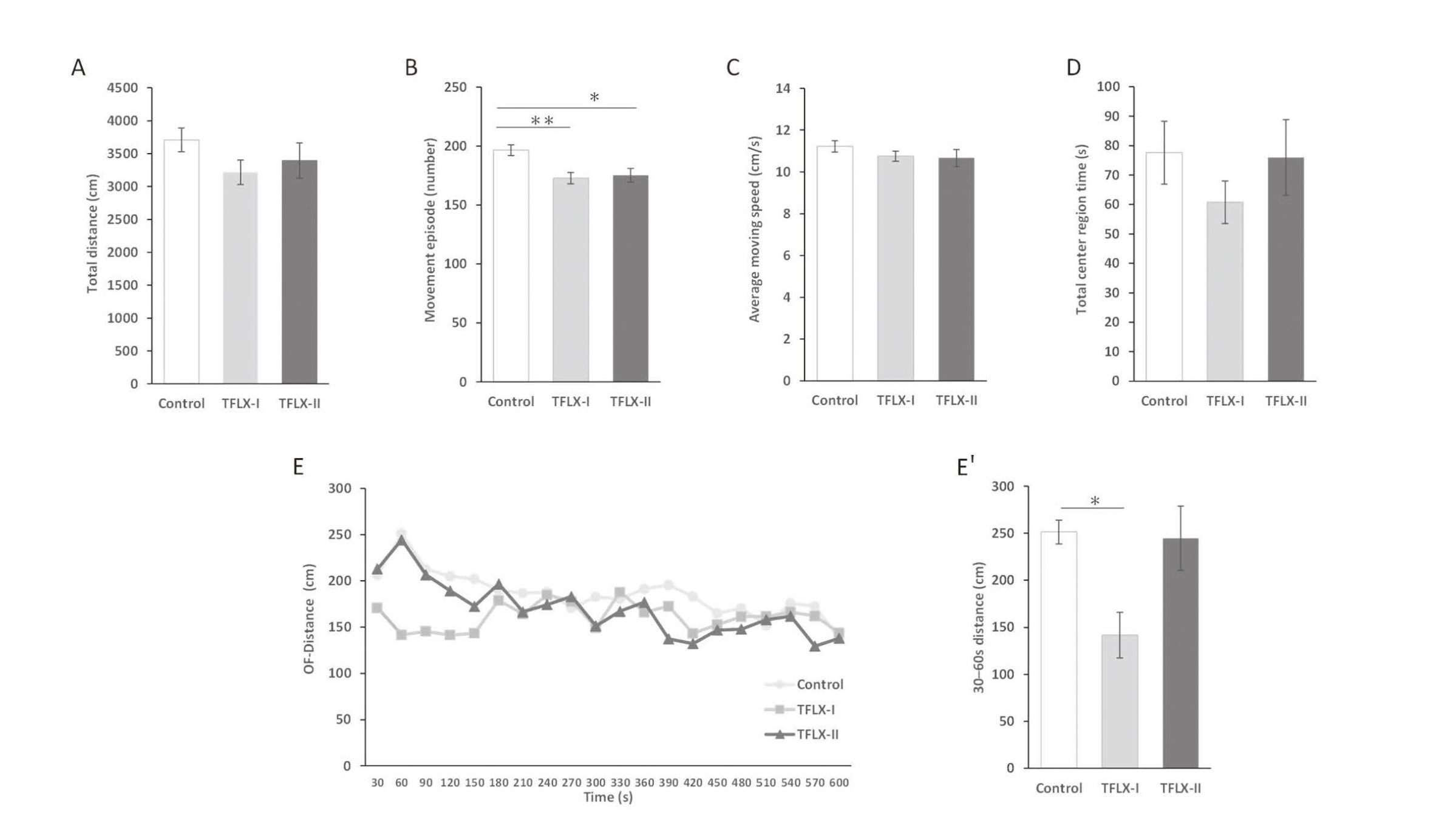
Open field (OF) test scores. A: Total distance traveled (cm) during the test time (600 sec). B: Total number of movements. C: Average movement speed (cm/sec). D: Total time spent in the central area (30% of the field) (sec). E: Temporal distance traveled (cm) during the test time. E′: Total distance traveled (cm) between 30–60 sec. Data were analyzed using Dunnett’s multiple comparison test and are expressed as the mean ± S.E. *P < 0.05, **P < 0.01 compared with the control group (n = 10 per group).
The results of the LD test suggested that the light area distance and time tended to decrease in the TFLX-I group compared with the control group (light area distance: control, 476.1 ± 24.8 cm; TFLX-I, 339.5 ± 42.9 cm; TFLX- II, 490.3 ± 56.3 cm; light area time: control, 84.0 ± 5.7 sec; TFLX-I, 59.6 ± 8.13 sec; TFLX-II, 87.2 ± 9.1 sec) (Fig. 4A and B, respectively). Notably, the light distance at 60–120 sec was significantly decreased (control, 104.2 ± 18.7 cm; TFLX-I, 44.4 ± 13.2 cm; TFLX- II, 101.5 ± 13.1 cm) (Fig. 4E, 4E′). In contrast, no significant differences were observed between the TFLX- II and control groups in this regard.
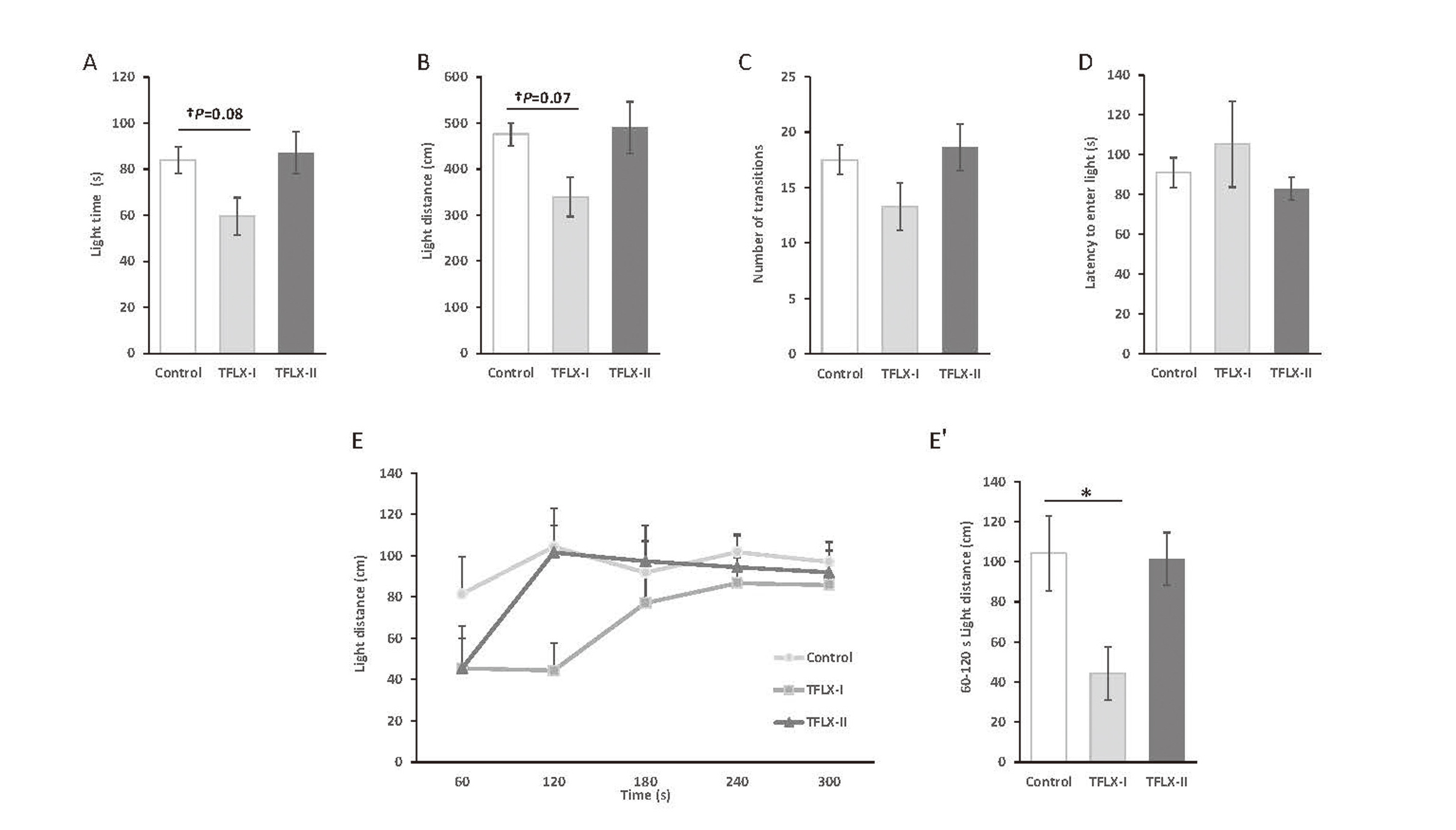
Light/dark (LD) transition test scores. A: Total distance traveled in the light chamber (cm). B: Time spent in the light chamber (sec). C: Total number of transitions between the light and dark chambers. D: Latency for the first move into the light chamber (sec). E: Temporal distance traveled in the light chamber (cm) during the test (300 sec). E′: Total distance traveled (cm) between 60–120 sec. Data were analyzed using Dunnett’s multiple comparison test and are expressed as mean ± S.E. † < 0.1, *P < 0.05 compared with the control group (n = 10 per group).
The FZ test indicated that mice in both the TFLX administration and control group exhibited an increase in their freezing response rate as the conditioning cycles were repeated (Fig. 5A). This indicated that learning was established in all the groups. The contextual fear test showed that mice in the TFLX-I group had a delayed initial freezing response compared with those in the control group. They also showed different behavioral tendencies (Fig. 5B). However, the cued fear test revealed, no differences between the TFLX administration groups and the control group (Fig. 5C).
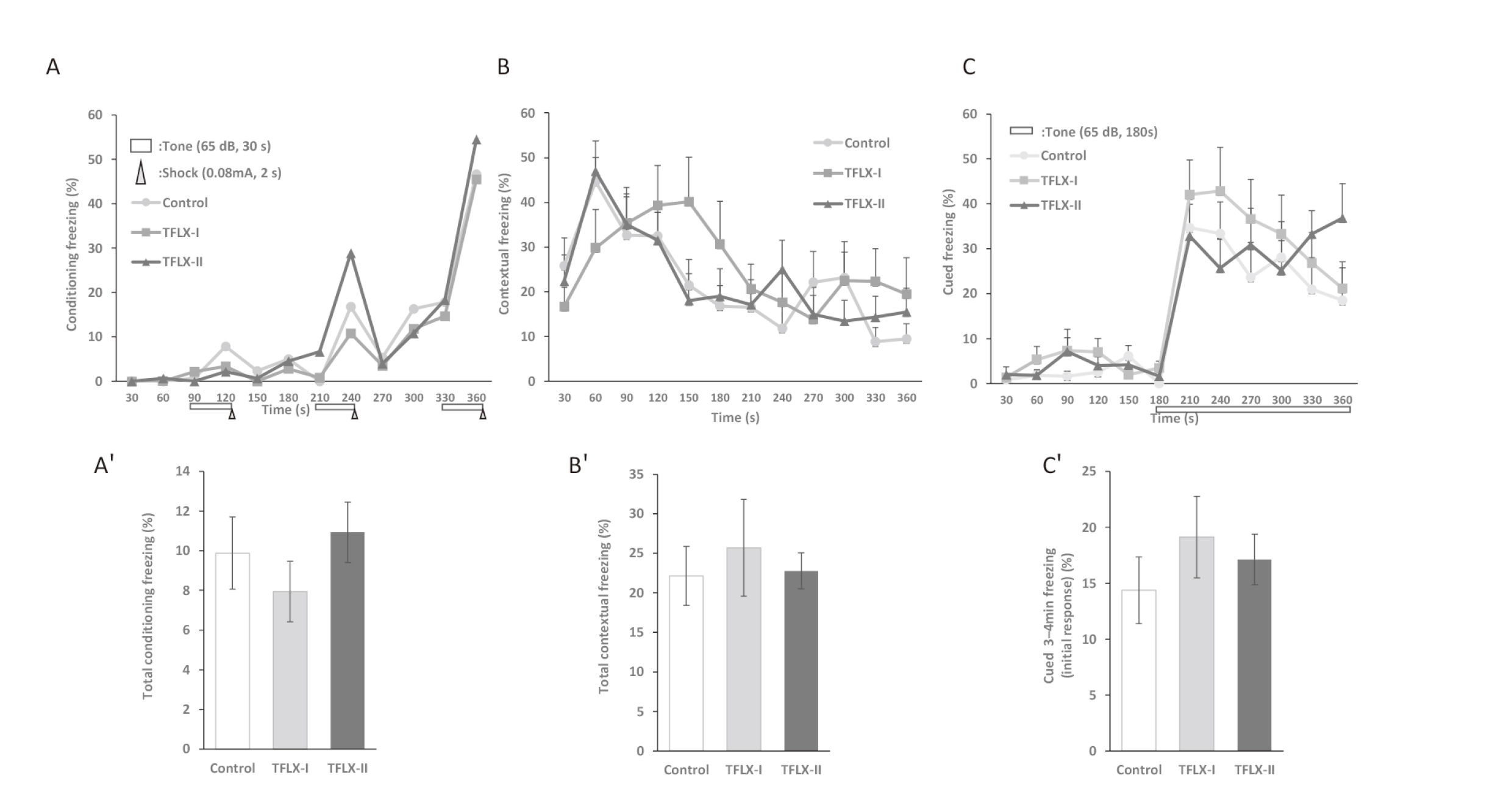
Contextual/cued fear conditioning (FZ) test scores. A: Conditioning test results. a, Time course of freezing scores (%) in the conditioning test. a′, Average total freezing score (%) in the conditioning test. B: Contextual test results showing the effects of place memory function. b, Time course of freezing scores (%) in the contextual test. b′, Average total freezing score (%) in the contextual test. C: Cued test results showing the effects of treatment on cued memory function. The tone was presented to the mice during the later period of the test (180–360 sec). c, Time course of freezing scores (%) in the cued test. c′, Average total freezing scores (%) for the first 1 min after the tone as an initial response to the tone in the cued test. Data were analyzed using Dunnett’s multiple comparison test and are expressed as mean ± S.E. (n = 10 per group).
The results of the behavioral test battery are summarized in Fig. 6 and Table 2.
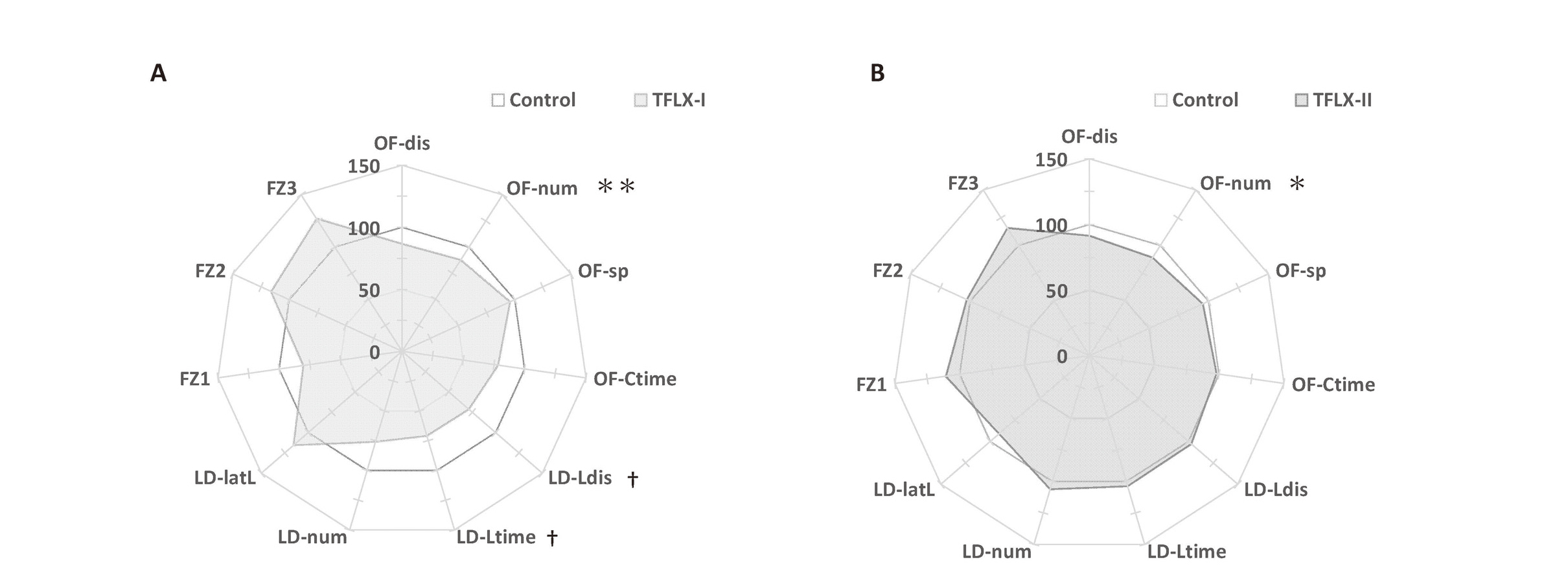
Summary of the mouse behavioral battery. The results of each item in the behavioral test battery are presented in a radar chart as the difference between the TFLX-I (A) and TFLX-II (B) groups against the control group. Each item was standardized based on the average scores of the control group mice, which s are represented as 100% in the radar chart. Data were analyzed using Dunnett’s multiple comparison test and are expressed as the mean ± S.E. *P < 0.05, ***P < 0.001 compared to the control group (n = 10 per group). OF (open field test): OF-dis, total distance; OF-Ctime, total center region time; OF-sp, average moving speed; OF-num, movement episode number; LD (light/dark transition test): LD-Ldis, light distance; LD-Ltime, light time; LD-num, number of transitions; LD-latL, latency to enter the light region; FZ (contextual/cued fear conditioning test): FZ1, total conditioning freezing; FZ2, total contextual freezing; FZ3, cued 3–4 min freezing.
| Test name | Behavioral parameters | Control | TFLX-I | TFLX-II | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Open field | Total distance (cm) | 3707.64 | ± | 179.43 | 3217.29 | ± | 185.26 | 3395.50 | ± | 269.78 |
| Movement episode no. | 196.70 | ± | 4.56 | 172.70 | ± | 4.80** | 175.30 | ± | 5.84* | |
| Moving speed (cm/sec) | 11.22 | ± | 0.27 | 10.75 | ± | 0.24 | 10.67 | ± | 0.41 | |
| Total center region time (sec) | 77.60 | ± | 10.70 | 60.75 | ± | 7.28 | 76.00 | ± | 12.83 | |
| Total movement duration (sec) | 297.00 | ± | 10.52 | 264.55 | ± | 14.06 | 281.65 | ± | 18.26 | |
| Average speed (cm/sec) | 6.18 | ± | 0.30 | 5.36 | ± | 0.31 | 5.66 | ± | 0.45 | |
| Distance per movement (cm) | 17.01 | ± | 0.78 | 16.46 | ± | 0.83 | 17.12 | ± | 1.16 | |
| Duration per movement (sec) | 1.51 | ± | 0.04 | 1.53 | ± | 0.06 | 1.60 | ± | 0.07 | |
| Light/dark transition | Dark distance (cm) | 1146.25 | ± | 32.31 | 1211.67 | ± | 53.50 | 1123.39 | ± | 60.18 |
| Light distance (cm) | 476.08 | ± | 24.77 | 339.468 | ± | 42.88† | 490.30 | ± | 56.30 | |
| Dark time (sec) | 276.05 | ± | 5.72 | 300.40 | ± | 8.13 | 272.85 | ± | 9.11 | |
| Light time (sec) | 83.95 | ± | 5.72 | 59.60 | ± | 8.13† | 87.15 | ± | 9.11 | |
| Number of transitions | 17.50 | ± | 1.33 | 13.30 | ± | 2.14 | 18.60 | ± | 2.10 | |
| Latency to first move (sec) | 91.00 | ± | 7.42 | 105.25 | ± | 21.56 | 82.95 | ± | 5.70 | |
| Fear conditioning | Total conditioning freezing (%) | 9.88 | ± | 1.82 | 7.95 | ± | 1.53 | 10.93 | ± | 1.52 |
| Total contextual freezing (%) | 22.15 | ± | 3.72 | 25.70 | ± | 6.10 | 22.78 | ± | 2.28 | |
| Cued tone 3–4 min freezing (initial response) (%) |
34.01 | ± | 7.34 | 42.43 | ± | 8.74 | 29.25 | ± | 6.74 | |
| Total cued tone freezing (%) | 26.54 | ± | 5.50 | 33.80 | ± | 6.31 | 30.77 | ± | 4.45 | |
Data were analyzed using Dunnett’s multiple comparison test and are expressed as the mean ± S.E.
† < 0.1, *P < 0.05, **P < 0.01 compared with the control group (n = 10 per group).
Neuronal loss or damage was examined via histopathological analysis using H&E hippocampal staining. Normal morphology was observed for mice in the control, TFLX-I and TFLX-II groups, i.e., no abnormalities were observed for the different groups. Further, immunohistochemical analysis using anti-NF, MAP2, α-tubulin, DCX, SOX2, BDNF, and GAD showed similar staining images for the control, TFLX-I, and TFLX-II groups, with no differences in protein expression patterns in the hippocampus (data not shown).
Gut microbiotaGroup differences were observed with respect to relative bacterial proportions at the phylum and genus levels (Fig. 7A, B). The percentages of the phyla Proteobacteria, Firmicutes, Deferribacteres, and Bacteroides did not differ between the different groups. However, the relative abundance of the phyla Actinobacteria was significantly decreased in mice in the TFLX-II group relative to the observed level for the control group mice (control, 0.057 ± 0.006; TFLX-I, 0.032 ± 0.004; TFLX-II, 0.018 ± 0.006). Further the TFLX administration groups showed lower Tenericutes relative abundance than the control group (control, 0.28 ± 0.07; TFLX-I, 0 ± 0; TFLX-II, 0 ± 0) (Fig. 7A′a and Fig. 7 A′b, respectively). Phylum Tenericutes was completely absent in mice in all the TFLX administration groups, whereas it was present in all the mice in the control group. The percentages of Turicibacter, Sutterella, Staphylococcus, Roseburia, Proteus, Oscillospira, Mucispirillum, Massilia, Lactococcus, Lactobacillus, Dorea, Desulfovibrio, Dehalobacterium, Coprococcus, Candidatus_Arthromitus, Butyricicoccus, Bilophila, Bacteroides, Agrobacterium, and Adlercreutzia genera were not different between the groups. However, compared with the control group, the TFLX administration groups showed decreased relative abundance of following genera: Anaeroplasma (control, 0.28 ± 0.07; TFLX-I, 0 ± 0; TFLX-II, 0 ± 0), Clostridium (control, 0.43 ± 0.05; TFLX-I, 0.14 ± 0.02; TFLX-II, 0.05 ± 0.01), Prevotella (control, 12.70 ± 0.80; TFLX-I, 3.80 ± 0.63; TFLX-II, 4.12 ± 0.47), and Ruminococcus (control, 2.23 ± 0.09; TFLX-I, 0.86 ± 0.08; TFLX-II, 0.59 ± 0.08) (Fig. 7B′a, Fig. 7B′b, Fig. 7B′c, and Fig. 7B′d, respectively). The Anaeroplasma genus was absent in all the TFLX-treated mice, but was present in all the mice in the control group. We also observed that the percentage of Parabacteroides was higher in the TFLX administration group than in the control group (control, 0.089 ± 0.05; TFLX-I, 2.47 ± 0.40; TFLX-II, 2.14 ± 0.23) (Fig. 7B′e).
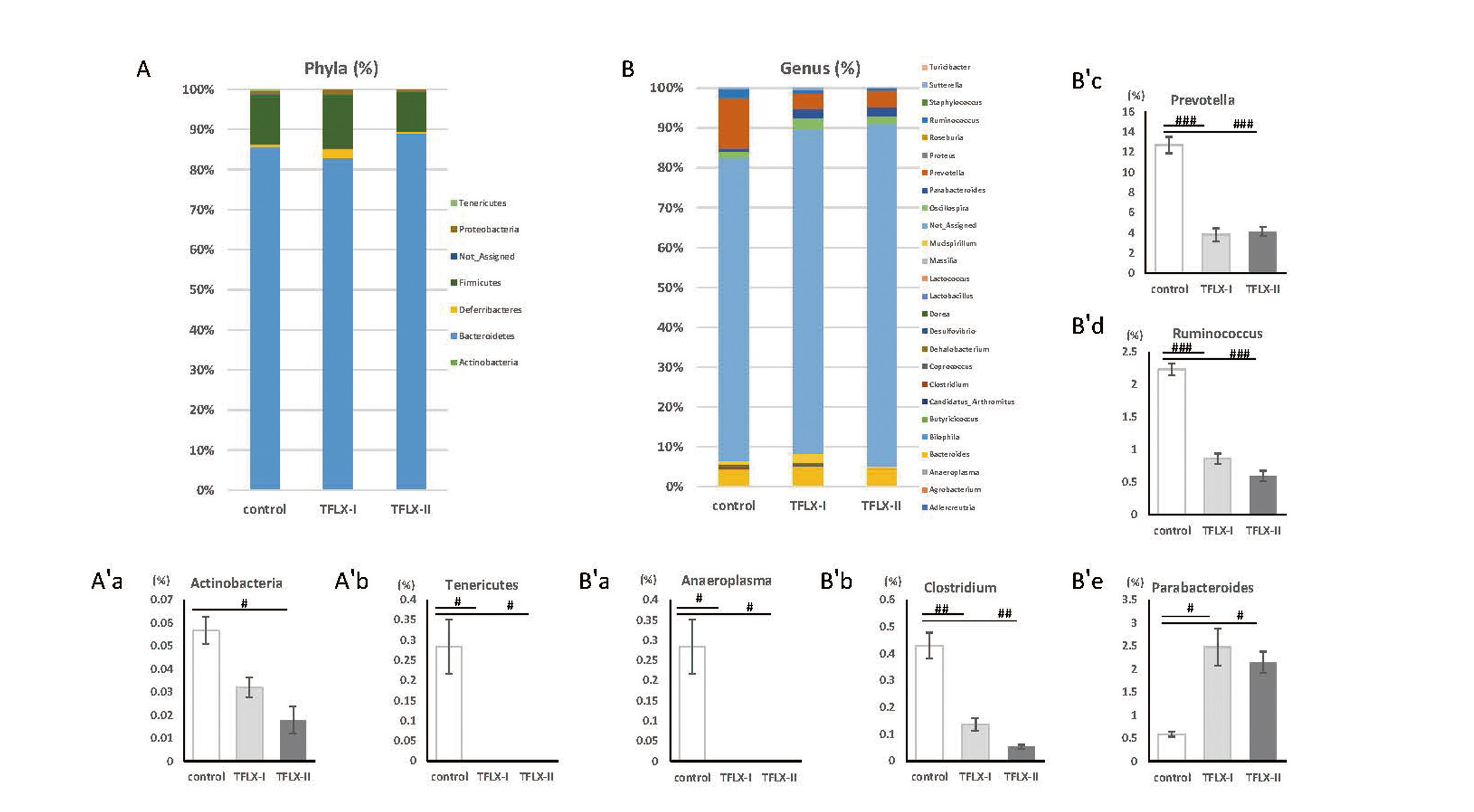
Microbiota analysis. A: Microbial composition at the phylum level expressed as percentages. A′: Relative abundance of the major phyla. A′a, Actinobacteria. A′b, Tenericutes. B: Microbial composition at the genus level expressed as percentages. B′: Relative abundance of the major genera. B′a, Anaeroplasma. B′b, Clostridium. B′c, Prevotella. B′d, Ruminococcus. B′e, Parabacteroides. Data were statistically tested using the Tukey–Kramer multiple comparison test and are expressed as the mean ± S.E. #P < 0.05, ##P < 0.01, ###P < 0.001 compared with the control group (n = 3 per group).
The administration of antibiotics during childhood can have long-term adverse effects via the disruption of the gut microbiota. Tosufloxacin tosilate hydrate (TFLX) has been approved for pediatric use in Japan. However, studies on its effects are limited. Therefore, in this study, we investigated the later effects of its administration during the developmental period based on changes in body weight, behavioral effects, and microbiota composition.
The group that received TFLX during the developmental period showed significant weight gain after the administration of drug compared with in the control group. Furthermore, TFLX administration altered the gut microbiota composition. This observation is comparable with previously reported results, suggesting that low-dose antibiotics administration to male mice at a young age can cause changes in gut microbiota composition and metabolic mechanism, resulting in obesity (Cho et al., 2012). Prevotella, which was significantly reduced following TFLX administration in our study, is a major SCFA-producing bacterium (Chen et al., 2017); SCFAs are closely related to metabolism, including glucose tolerance (Kimura et al., 2013; Chambers et al., 2015). Similarly, in this study, we also observed that Prevotella was significantly reduced in the TFLX-treated group, suggesting that metabolic changes may have been induced by changes in the gut microbiota, as shown in previous studies. The difference between the results of this study and those reported by Furubo et al. (2010), in which no weight gain was observed despite TFLX administration starting at 7 days of age, might be due to different age having different gut microbiota structures (Mitsuoka, 2014). The gut bacterial composition of the TFLX-I group (6 weeks after administration) and the TFLX-II group (2 weeks after administration) exhibited minimal difference suggesting the persistent effects of TFLX on gut bacterial. However, body weight differences between the TFLX-I and control groups decreased over time, suggesting that the effects of TFLX on metabolism did not persist. Early low-dose antibiotics induce long-term obesity via changes in gut microbiota (Cho et al., 2012), implying that metabolic changes resulting from changes in gut microbiota structure persist. However, counterintuitive results were obtained in this study. Metabolic systems are highly homeostatic. Further, hypernutrition generates in neural signals and leptin signaling from the white adipose tissue to promote a negative-feedback mechanism of appetite suppression and increased heat production (Rosen and Spiegelman, 2006; Yamada et al., 2006). In our study, the effect of this system was greater than that of gut microbiota changes. Thus, this system may have suppressed the long-term effects of TFLX on metabolism.
Mice in the group that received TFLX during adulthood demonstrated a significant decrease in the number of movement episodes in the OF test, while those in the group that received TFLX during the developmental period exhibited a substantial decrease in the number of movement episodes in the OF test and also showed a trend towards a decrease in the distance covered and time spent in the light area of the LD, which offers the possibility to measure anxiety-like behavior. Furthermore, we also observed a significant decrease in the initial response (i.e., the distance traveled over time under the OF and LD conditions). These findings suggested that anxiety may have increased following TFLX treatment. No significant difference was observed between the TFLX-treated groups and the control group in the FZ. Based on the contextual fear test, we observed a delayed increase in the freezing response rate of mice in the TFLX-treated group during the developmental period and qualitative differences in memory recall patterns. This possibly indicated the absence of any abnormality in memory formation or recall, but may indicate a modulation of memory recall. These findings suggested that TFLX has behavioral effects when administered during the developmental period.
It is unclear whether this behavioral effect directly resulted from TFLX administration, and there may be some possible limitations in this study. However, spinal fluid transfer of TFLX after administration is low in humans (Inatsuchi et al., 1993). TFLX at 50 mg/kg administered orally in rats has little to no migrate into the brain, suggesting that TFLX has little blood-brain barrier migration (Maeda et al., 1989). Therefore, the influx of TFLX into the brain is unlikely. In addition, morphological and immunohistochemical analysis of the hippocampus showed no nerve loss as well as the absence of abnormalities in axonal elongation or neurogenesis. These findings suggested that the direct effects of TFLX to the hippocampus may be minor and other factors may be involved. Antibiotics have long-term effects on higher brain function and behavioral patterns (Leclercq et al., 2017; Volkova et al., 2021). These phenomena are thought to occur via the disruption of the gut microbiota. For example, changes in the relative abundance of gut Ruminococcus induce anxiety-like behavior mediated by inflammatory cytokines (Bangsgaard Bendtsen et al., 2012). Our findings showed Ruminococcus was significantly reduced in the TFLX-treated groups, suggesting that changes in bacterial flora might be a factor in inducing behavioral changes. The underlying mechanism remains unclear; hence, future studies on homeostatic changes including changes in endocrine-, immune-, and neural responses, are necessary. Additionally, the relationship between the gut bacteria and behavioral changes in our study also requires further examination, including the elucidation of intervening factors.
In this study, the group that received TFLX during the developmental period exhibited greater weight gain as well as greater degree of behavioral abnormality compared with the group that was administered TFLX at the adult stage. Therefore, as previously reported, the developmental period is critical for metabolic development (Knittle and Hirsch, 1968; Cunningham et al., 2014). Further, exposure to chemicals and drugs during the developmental period interferes with brain function development and induces delayed effects (Rice and Barone, 2000; Saito et al., 2019). This is believed to occur because the developmental period is a critical stage in the development of neural pathways and establishes irreversible effects. The effects of early antibiotic administration on weight gain and behavior observed in this study may be due to the high sensitivity of the animal during the developmental period.
Using human equivalent dose (HED) with body surface correction factors for the 300 mg/kg TFLX used in our study (17 g mice and 10 kg pediatric patients) (U.S. Food and Drug Administration, 2005), we estimated that this dosage corresponds to 365 mg in humans, slightly higher than the established maximum intake of 360 mg for pediatric patients. Therefore, it would be difficult to directly extrapolate all the data obtained in mice to humans. However, this study is the first to suggest that TFLX administration has potential long-term risks. The present study using mice should be translated to further studies in humans to determine the negative effects of administering TFLX during the developmental period. Further, our findings may be valuable in assessing the risk of administering TFLX at an early age and clarifying the mechanism of its toxicity.
The results of this study suggest that TFLX administration during the developmental period affects the growth rate, behavior, and gut microbiota composition after administration. However, its comprehensive relevance remains unclear and requires further research to identify intervening factors. Further, assuming that other antibiotics have similar intervening factors, it is therefore critical to consider the potential risks of other drugs when prescribing antibiotics to children.
We thank Dr. Saito for helpful discussions regarding the preparation of the behavioral experiments. We are grateful to Editage (www.editage.com) for the English language editing. This study was supported in part by Health Sciences Research Grants from the Ministry of Health, Labour, and Welfare, Japan (Grant Number H30-KAGAKU-IPPAN-003), and JSPS KAKENHI (Grant Number 19H01142).
Conflict of interestThe authors declare that there is no conflict of interest.